Manufacturing/Importation Authorization for medicinal products
We have a Manufacturing/Importation Authorization for medicinal products, as well as a Authorization to process, repackage, import and certify medicinal products containing group II-P, III-P and IV-P psychotropic substances as well as group IN, II-N, and III-N narcotic substances.
- We also provide repackaging services for medicinal products, dietary supplements, cosmetics and food products.
- We can assemble promotional packages, displays, stands, and the like.
- We are constantly improving our processes to guarantee fast order fulfillment and top quality products, and we promptly adapt our actions to the specific client's requirements.
- We also offer logistics support with every order.

Medezin Sp. z o.o. Repackaging Facility See the movie
Packaging and repackaging
We pack and repack medicinal products!
The scope of our services includes processing unit packaging and/or direct packaging without any contact with the product (“secondary packaging”) - for:
- medicinal products, including products containing group II-P, III-P and IV-P psychotropic substances and group IN, II-N and III-N narcotic substances,
- dietary supplements,
- medical devices,
- cosmetics,
- food products.
We also provide a visual inspection service for non-compliance:
- detect tampering with the packaging, closures or protections,
- color differences,
- compliance of the contents with the amount declared on the box.
We work in accordance with the principles of Good Manufacturing Practice (GMP) and the HACCP system.
What do we offer?
What do we offer?
- we can adapt the language on packaging materials,
- we can change the size of a unit package,
- we can label unit packaging or direct packaging, and help you create, design and purchase packaging materials.
For:
Medicinal product serialization
We offer a full range of labeling options for medicinal products!
We provide a full range of options for the labeling and repackaging of medicinal products subject to serialization (FMD - Directive 2011/62 / EU, known as the “Falsified Medicines Directive”). The serialization of medicines and medicinal products allows you to verify the authenticity of the product and identify the packaging of the medicine, thus preventing the introduction of falsified medicines into the legal distribution chain.
Since February 9, 2019, medicine manufacturers have been required to provide two types of protection on medicine packages, so-called “safety features”, which include the following:
- unique identifier in the form of a 2D code (UI),
- anti-tampering device (ATD).
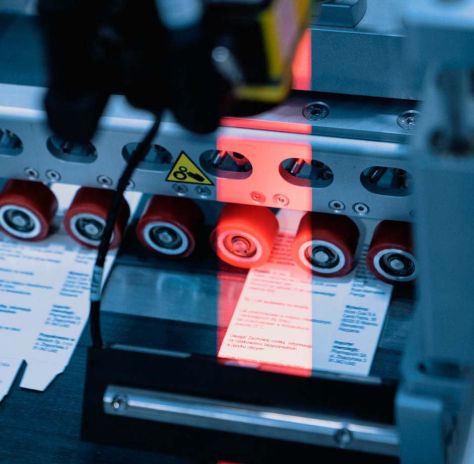
- Each package of a serialized product is repacked by us in accordance with applicable legal requirements.
- We conduct a 100% inspection of the correctness, quality and legibility of the data placed on the products.
- Safety features are applied in accordance with specific customer requirements and meet specific legal regulations.
Printing on packaging and labeling
We provide printing and labeling for medicinal products!
Special printers designed for our specific needs guarantee top quality printing on unit packages of products repackaged at our facility.
These printing devices enable us to apply the required data identifying the product to the unit packages, including the batch number, expiration date, PC/GTIN code and the 2D Data Matrix code.
We provide labeling for unit packaging and direct packaging of products supplied by the customer. We can also mark products as “Free sample - not for sale”.
Thanks to our machinery and the knowledge of our experienced staff, we can repackage virtually any product for which labels are needed.
Our machine park is made up of the following:
- Intrex Pharma carton feeder (high resolution prints, including Data Matrix),
- Domino printer (inkjet) for prints on direct packaging,
- Domino G-series printer with carton conveyor.
Kits and promotional campaigns
We put together promotional campaigns and kits, for business offers or for holiday events!
Our business activity is not limited to pharmaceutical companies only. We also cooperate with the FMCG market, retail chains and other broadcasters, by offering a packet of services broadly understood as co-packing.
We prepare sales kits, displays, stands or other types of promotional packaging in accordance with the client's requirements. We assemble products in promotional kits for business offers or for holiday events.
We can custom package your products.
With our co-packing services, we can:
- prepare promotional campaigns, assemble products into sets according to client requirements,
- prepare displays and stands according to client requirements,
- package holiday sets, gifts and sets for special occasions,
- apply labels to round products, such as bottles or cans,
- apply custom packaging and inserting according to client requirements.

Quick promotional campaign

An unconventional order

Express delivery date

Timeliness and reliability

Competitive prices

Professional pricing quote
Medezin - zapytaj o ofertę
- We help our clients launch promotional campaigns and adapt the appearance, size and layout of the kit/packaging to optimize the costs of the packaging process.
- We also offer logistics support for promotional campaigns.
Storage of production components
We offer storage
- We have a high-bay warehouse with plenty of space to store large amounts of production components.
- The facility also has a designated area for products imported from outside the EU (and EFTA) - the Importer's Warehouse.
- We have separate storage spaces for both raw materials and packaging materials.
- We provide controlled temperature and humidity conditions throughout the entire area.
A validated air temperature and air humidity monitoring system has been installed in all storage and production rooms to ensure appropriate process conditions.
Certification and release of products
We certify and release products
We provide a full range of services related to the certification and release of medicinal products by Qualified Persons for circulation or further distribution.
Batch Certification means that positive verification has been made that a product was manufatured and inspected in accordance with applicable law and authorized for release for circulation, or for conducting clinical trials (with consideration of Good Manufacturing Practice - GMP).
An experienced team confirms the quality of repackaged medical devices, dietary supplements, cosmetics and food products.

Logistics services
We will transport the products wherever you need them to go!
We offer the transport of products to and from the client warehouse or directly to designated destinations, using a fleet of vehicles ensuring transport in controlled temperature conditions (GDP standards). We offer logistics support for our clients as part of our comprehensive project management services.
Our production facility is located right in the center of Poland, near the intersection of the key A1 (north-south) and A2 (east-west) motorways. The location is the optimal starting point for the ongoing journey of customer orders throughout Poland and abroad.






